Two-Dimensional 2D organic-inorganic hybrid perovskites which have a general formula of L 2 PbX 4 L is a large aliphatic or aromatic amine cation X is a halide anion have attracted extensive interest because of their excellent performance in photovoltaics and other optoelectronic devices Due to the dielectric enhancement effect and quantum confinement effect. Called its λmax pronounced lambda max and can be measured by UV-visible spectroscopy.
Comparative Dataset Of Experimental And Computational Attributes Of Uv Vis Absorption Spectra Scientific Data
Uv Visible Spectroscopy

Detected Shifts In Absorbance Maximum L Max In Response To Enzyme Download Scientific Diagram
Shows the deviations in Beer-Lambert law due to observations made at.

Relationship between absorbance and lambda max. Keep this quantity in mind. However there is usually a disordered layer between the substrate and ordered ZnIn 2 S 4 ZIS nanostructures which lowers the bulk separation efficiency η sep of the photoanode 161718. The instrument used in ultravioletvisible spectroscopy is called a UVVis spectrophotometerIt measures the intensity of light after passing through a sample and compares it to the intensity of light before it passes through the sample The ratio is called the transmittance and is usually expressed as a percentage TThe absorbance is based on the transmittance.
Hopkins WHuner N-Introduction to plant physiology-2008pdf. This is in the ultra-violet and so there would be no visible sign of any light being absorbed - buta-13-diene is colourless. You will be able to determine which chemical dye was used in the solution.
What you want to do is. There is a fundamental relationship Gustav Kirchhoffs 1859 law of thermal radiation that equates the emissivity of a surface with its absorption of incident radiation the absorptivity of a surfaceKirchhoffs law explains why emissivities cannot exceed 1 since the largest absorptivitycorresponding to complete absorption of all incident light by a truly black objectis also 1. The relationship between absorbance and transmittance is illustrated in the following diagram.
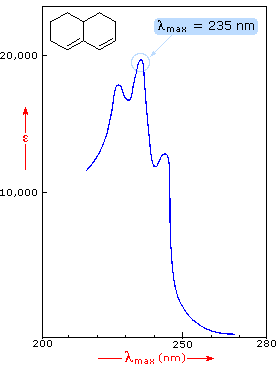
9C shows the spectrum over the range subject to most change at the Pt gauze electrode pre and post cycling with λ. Shows the difference in deviations in absorbance when values are obtained at maximum wavelength of absorbance band A vs other wavelengths of absorbance band B. You will see that absorption peaks at a value of 217 nm.
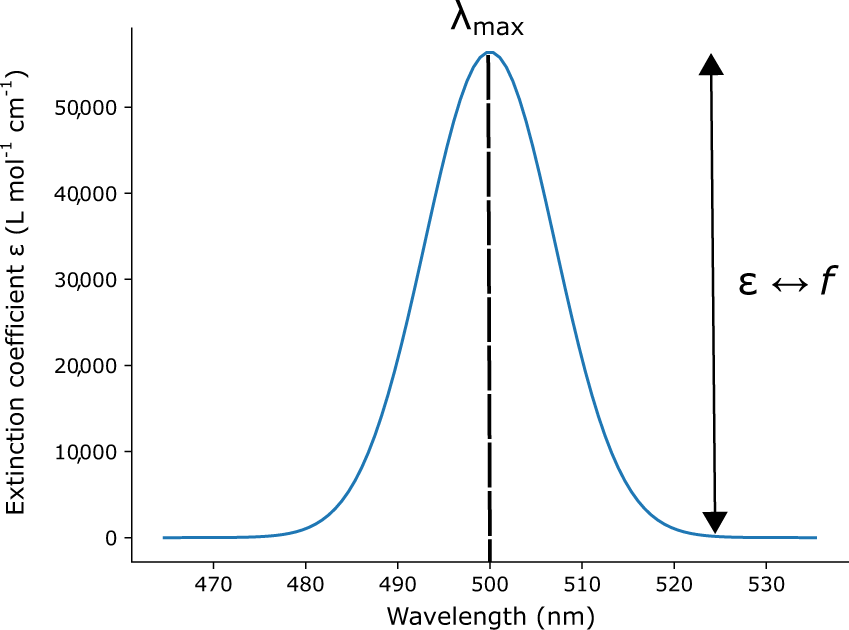
Lambda Max 575 nm A maximum in absorbance occurs at 575 nm. The wavelength that has the highest absorbance in the spectrum is lambda max. What is Beer and Lamberts Law Molar absorptivity Molar Extinction coefficient E 1 lambdaMax How to calculate from Absorbance.
This spectrum lambda max is 400 nm. Usually the more concentrated a substance the more light will be absorbed. Full PDF Package Download Full PDF Package.
You have 1s and oftentimes with wavelength you want to convert to nm which is UV-Vis range 200700 nm and is often of spectral interest. The absorption cross-section and BlambdaT terms found in the Schwarzschild eqn making it moe fundamental than Planck and SB. 37 Full PDFs related to this paper.
If you note the units you are using it becomes easier. So if all the light passes through a solution without any absorption then absorbance is zero and percent. Lambda max is the wavelength at which the maximum fraction of light is absorbed by a solution.
Lambda is a Greek letter that scientists use as the symbol for wavelength. Practically speaking its what youre the most interested in. A short summary of this paper.
You read the symbol on the graph as lambda-max. Applied predictive modeling max kuhn kjell johnson. A log T Equation 4 In older designs of the spectrophotometer the T scale is much easier to read because it is linear as compared to the A scale which is logarithmic.
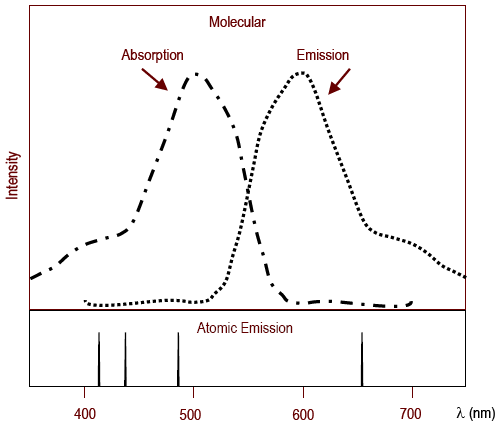
A single point measurement recorded at λ max will be used to calculate the concentration of red yellow green and blue food dyes in a solution. Sample absorption spectrum showing an absorption. Such an instrument is used to determine the relationship between a wavelength and color.
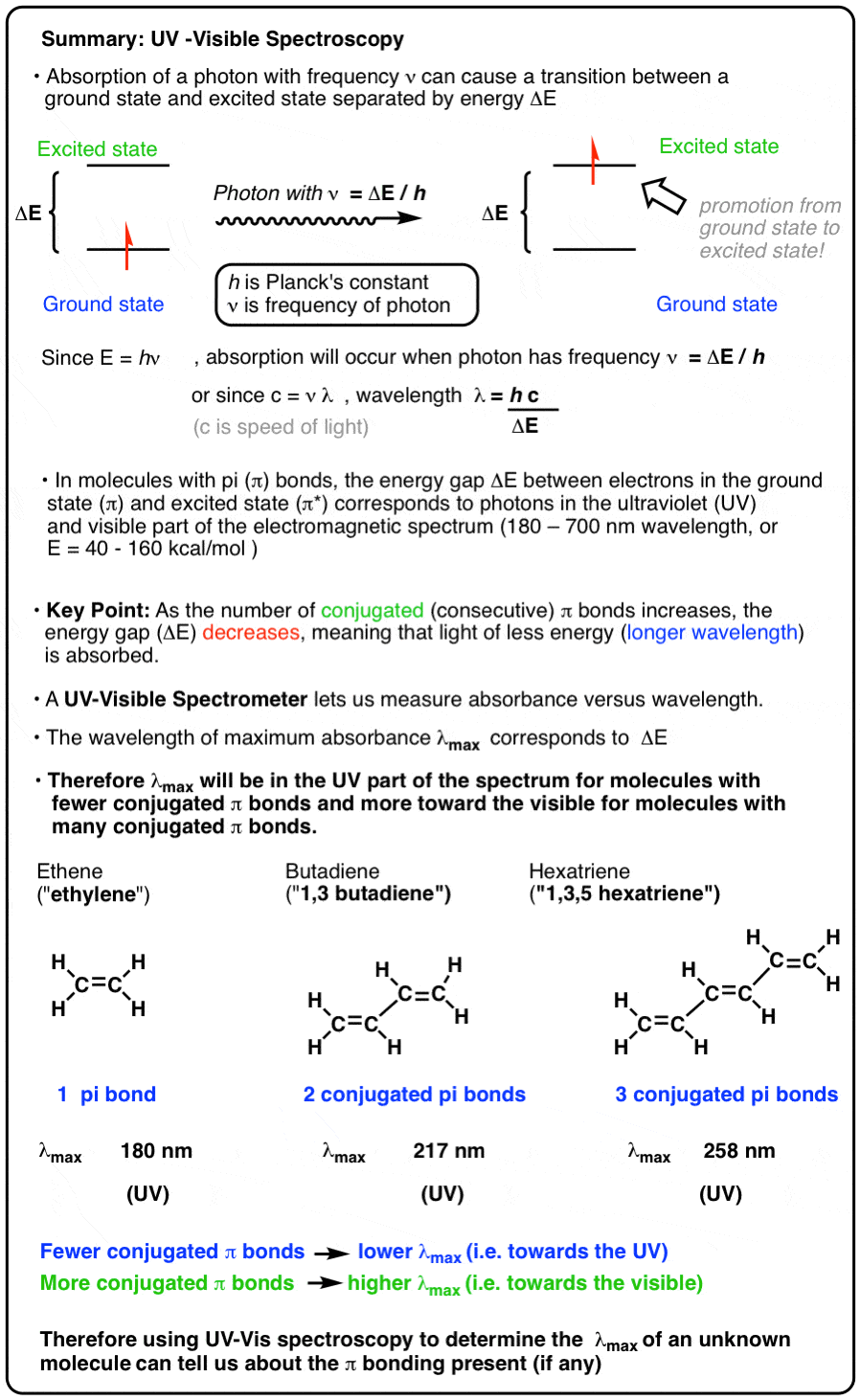
Applied predictive modeling max kuhn kjell johnson. The graph of absorbance versus concentration at a constant wavelength is called a. The relationship between HOMO-LUMO gap and absorbed wavelength of light.
How to calculate concentration of analyte from Absorbance using and Calibration curve. The relationship between absorbance and concentration c is proportional. The relationship between temperature and emission is as clear as it can be.
1s - 1m - m - nm Conversion factors are extremely useful and one easy one to remember is the speed of light which is about 3 xx 108 ms. Is transmittance directly proportional to absorbance. Because of the logarithmic relationship between absorbance and transmittance the absorbance values rise rather rapidly over the last 10 of the radiation that is absorbed by the sample.
9B presents the data in absorbance ratio format with relative changes post cycling being evident at regions I and II of the spectra Abs ratio 1 corresponding to 339 and 452 nm. The relationship between absorbance of a solution and the concentration of the analyte in the Beer-Lambert Law will be qualitatively and quantitatively investigated. The relationship between absorbance and percent transmittance is expressed by Equation 4.
This wavelength is used to determine the relationship between absorbance and concentration. Lambda max refers to the wavelength along the absorption spectrum where a substance has its strongest photon absorption. 1 IFTHENELSE 2 WHILEDO called by some DOWHILE 3 DOUNTIL 4 CASE Because of the lack of block structure it is necessary to use statement labels in the simulation of the standard figures.
Planck and Beers Law can be derived from Schwarzschild You are not very clear about what you are saying. You will see that absorption peaks at a value of 217 nm. In buta-13-diene CH 2 CH-CHCH 2 there are no non-bonding electrons.
Application in food analysis Understand the simple components of a UV-VIS spectrum. You read the symbol on the graph as lambda-max. Wavelength of Maximum Absorbance.
Make sure the spectrometer is set up to measure UV-Vis absorbance by. How to calculate unknown concentration from standard curve. Relationship between performance index on the absorption basis PI ABS and concentration of carbohydrates soluble carbohydrates and starch in the leaves of rose plants grown under different light spectra blue B red R white W and red and blue RB under 250 C and 1500 HL µmol m 2 s.
Lambda max is the wavelength in the absorption spectrum where absorbance hits maximum. Z 1 2 0. Scientists can then use lambda max as a parameter to compare the different qualities of all types of molecules and substances.
Converting percent transmittance to. Called the wavelength of maximum absorbance which is abbreviated to λ max spoken as lambda max. This is in the ultra-violet and so there would be no visible sign of any light being absorbed - buta-13-diene is colorless.
In buta-13-diene CH 2 CH-CHCH 2 there are no non-bonding electrons. In order to. Com In this section the conventions for simulating the following standard structure figures will be outlined.
Lab 9 Determination Of Allura Red Concentration In Mouthwash
What Is Uv Vis Spectroscopy And How Does It Apply To Conjugation
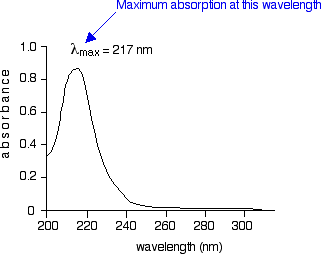
Uv Visible Absorption Spectra

Effect Of Ph On Absorbance L Max For Complexes Download Scientific Diagram
Lambda Max
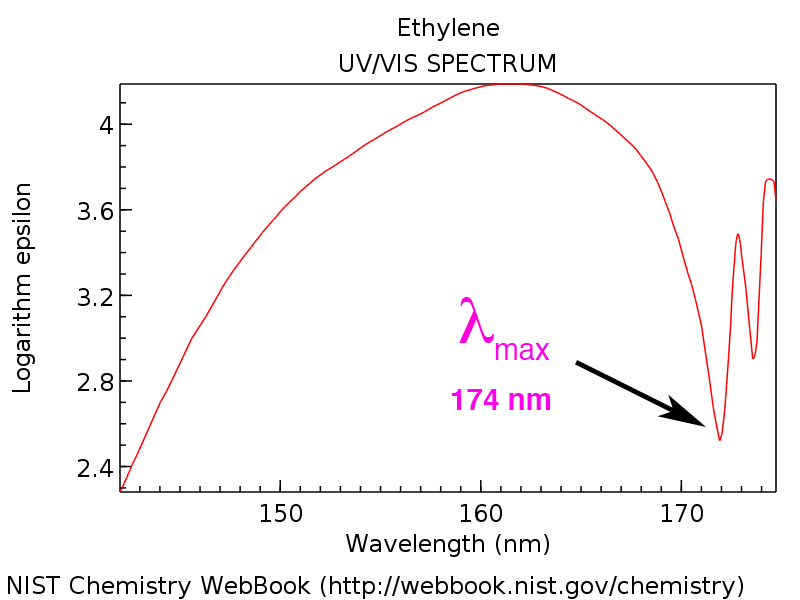
What Is Uv Vis Spectroscopy And How Does It Apply To Conjugation

What Is The Effect Of The Wavelength On The Spectrophotometric Measurements Of Protein Biology Stack Exchange

Beer Lambert Law Transmittance Absorbance Edinburgh Instruments
