NIR excitation at 1064 nm using either DPSS or Yb-doped fiber lasers VIS excitation at 532 nm produced by frequency-doubling a 1064 nm laser and SWIR excitation at 1550 nm using Er-doped fiber lasersEach wavelength has a unique set of advantages and disadvantages that depend on the target reflectance and absorbance background. Three different wavelength regions are used in LiDAR systems.
1

What Does A Decrease In Ftir Peaks Intensity Show

Near Infrared Spectroscopy Wikipedia
How Does Fluorescence Spectroscopy Work.

Spectra ftir higher absorbance. T and A spectra graphs are illustrated in Figs. Transmittance ranges from 0-100. Higher TEMPO doping concentration also resulted in the stronger light adsorption performance in PDA-i.
Since its frequency is close to the overtone frequency of many natural vibrations weak substance-specific absorption bands can be detected. It can be used to characterize new materials or identify and verify known and unknown samples. A CD spectra of poly-L-lysine at pH 111 in the 1 black α-helical and 2 red antiparallel β-sheet conformations and at pH 57 in the 3 green extended conformations 5 and placental collagen in its 4 blue native triple-helical and 5 cyan denatured forms 64.
Transmittance value of the powders is very low and T value is approximately 30 for the optical region. Circular dichroism CD spectra of polypeptides and proteins with representative secondary structures. Both are the same just 1 transmittance is absorbance.
A brief discussion of issues is presented below concerning the comparison of spectra in this collection with spectra acquired on modern FTIR instruments. The FTIR spectra were collected before and after the 5 days long heliumnitrogen mixture separation test. The present paper details on the synthesis and characterization of the modified Graphene and its moisture sensing application.
The size of these small spheres give quantum dots the semiconducting properties and resulting photoluminescence that would not necessarily occur for the same material on larger scales. The first is located at 7483 eV FWHM 190 eV corresponding to Al-O state and the second is located at 7230 eV FWHM 336 eV corresponding to Al-M state. Hence the name FT-IR or FTIR.
The springs vibrate and each one sings at a characteristic frequency which depends on the strength of the bond and on the masses of the. The FTIR spectra of fresh and aged CMS were compared and it was observed that a typical band at 1605 cm 1 ascribed to C C bond and a tiny band at. The infrared detector measures the amount of energy at each frequency that has passed through the sample.
Method 1 using fingerprint modes shows all the layers but the contrast is poorer particularly for higher numbers of layers. Not only is the acquisition of FT-IR spectra much faster than by conventional dispersive Instruments. It is used to detect different functional groups in PHB.
The Al2p spectra are shifted 1 eV to higher binding energy in the order α-Al 2 O 3 θ-Al 2 O 3 γ-Al 2 O 3. The interferometer is a fundamentally different piece of equipment than the monochromator found in UVVis spectrometers. Fourier transform infrared spectroscopy FTIR is a technique which is used to obtain infrared spectrum of absorption emission and photoconductivity of solid liquid and gas.
The characterization tools as BrunauerEmmettTeller BET Scanning Electron Microscopy SEM Electron Dispersive X -rays EDX Particle size distribution Fourier-Transform Infrared FTIR UVVis spectrophotometry and Raman are employed for characterizing. A double band is observed for the as-received PM2000. This had become possible by the availability of high signal-to-noise ratio digitalised spectra obtained by the FTIR spectrometer and by the access to computers and software able to perform many operations on the spectra in a short time.
The powders were transferred to an adhesive band. Single bond so higher energy input or higher frequency is needed to cause a vibration. The excitation energy provided to the molecule at the ground state promotes photons to an excited singlet state where they then decay to the lowest vibrational energy level of this excited singlet state.
Measurement of pulsed spectra suffers from several issues that must be overcome for accurate measurements. For instance spectral ghosts arise due to the pulsed nature of the source as well as the varying optical path difference OPD of the OSA. Example of FTIR spectra 1.
The spectrometer works by sending source energy through an interferometer and through the sample. How To Interpret IR Spectra In 1 Minute Or Less. 4a and 4b respectively.
All the measurements low-frequency and fingerprint were done using ultra-low frequency ULFTM filters which allow a high throughput measurement in a full Raman range down to. Quantum Dot Absorbance Photoluminescence Spectra and Lifetimes Quantum dots QDs are semiconducting spheres in the size typically in the range of 1 to 10nm. In every scan all source radiation goes through the sample.
For prism-based spectrometers the spectral resolution of a given prism material depends directly on the rate of change of its refractive index versus wavelength which in turn depends on wavelength. The instrument used in ultravioletvisible spectroscopy is called a UVVis spectrophotometerIt measures the intensity of light after passing through a sample and compares it to the intensity of light before it passes through the sample The ratio is called the transmittance and is usually expressed as a percentage TThe absorbance is based on the transmittance. Spectral intragroup variability and intergroup differences between the N LG and HG BC groups were presented in averaged FTIR spectra Figure 2 A and Figure S2 in SM as well as in the PCA loadings and score plots Figure 2 and Figure S3 in SM.
Similarly an H-Cl bond will vibrate at a different frequency than an N-O bond. Transmittance and absorbance spectra of C-BNNS powder were recorded in the wavelength range 2001100 nm. In addition in the UV-visiblenear-infrared UV-vis-NIR absorbance spectra of PDA samples PDA-i i 1 to 3 showed much stronger absorptions at both of visible and NIR regions compared to PDA-0.
Hi FTIR the spectra measured by instrument is transmittance and later its changed to absorbance by the software such as OPUS. This results in a spectrum which is a plot of absorbance. With a publication by Byler 253469-87 the determination of secondary structures in proteins from FTIR spectra actually started.
FTIR spectrum is recorded between 4000 and 400 cm 1For FTIR analysis the polymer was dissolved in chloroform and layered on a NaCl crystal and after. Although your software will show higher absorbance values. B CD spectra of.
Absorbance ranges from 0 to infinity. Fluorescence is a type of radiative emission that occurs when a molecule absorbs energy at a wavelength where it has a transition dipole moment. We recommend to take in account absorbance values 0-1 or maximally 0-2.
The best result can be obtained combining the two methods. A visual inspection of the mean FTIR spectra also suggested a higher content of carbohydrates in LG BC cells than in HG BC. UV-VisibleNIR spectroscopyUV-Vis Spectroscopy can be divided into ultraviolet visible and near-infrared regions of the spectrum depending on the wavelengths used.
Or Absorbance logarithmic scale peaks go up units. The FTIR or Fourier Transform Infrared Spectrometer. Additionally these spectra show a significantly higher signal-to-noise ratio and since the wavelength scale is calibrated with a very precise laser have much higher wavelength accuracy.
In addition the noise floor for pulsed sources is much higher than that for CW sources. The 2 Most Important Things To Look For Tongue and Sword Last post we briefly introduced the concept of bond vibrations and we saw that we can think of covalent bonds as a bit like balls and springs. Infrared spectroscopy IR spectroscopy or vibrational spectroscopy is the measurement of the interaction of infrared radiation with matter by absorption emission or reflectionIt is used to study and identify chemical substances or functional groups in solid liquid or gaseous forms.
This study is potential to be used as a standard information on how to read FTIR peaks in the biochemical and organic materials.
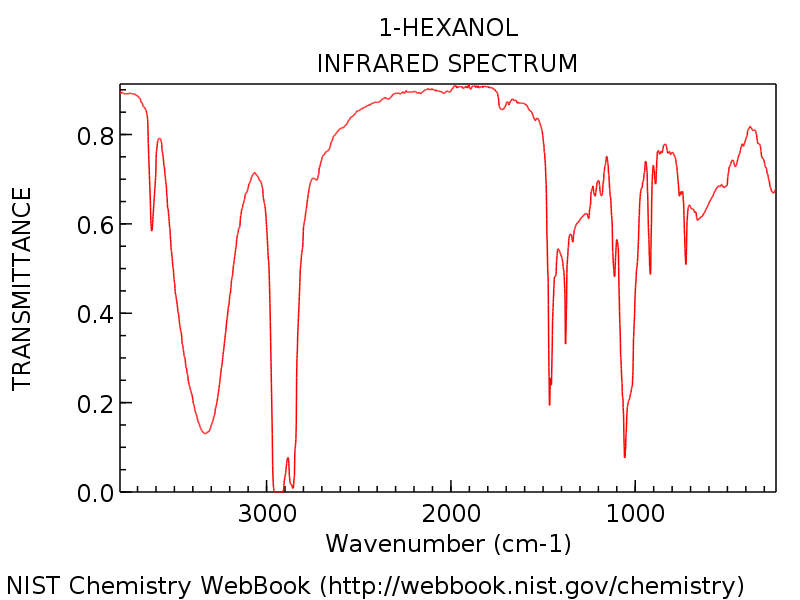
Interpreting Ir Specta A Quick Guide Master Organic Chemistry
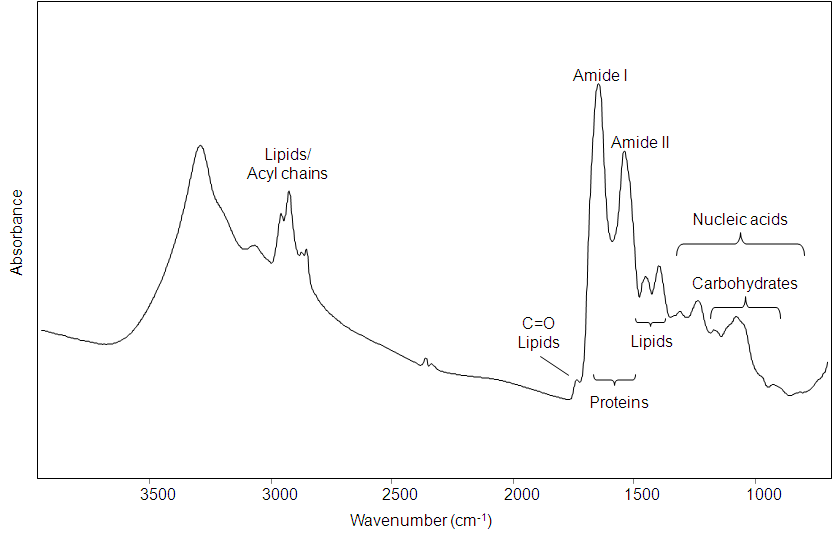
Multivariate Analysis For Fourier Transform Infrared Spectra Of Complex Biological Systems And Processes Intechopen

Broadband Laser Based Mid Ir Spectroscopy For Analysis Of Proteins And Monitoring Of Enzyme Activity Sciencedirect
.jpg)
Characterizing Diamonds With Ft Ir Spectroscopy
Fourier Transform Infrared Spectroscopy Wikipedia
.jpg)
Analyzing The Gas Phase Spectrum Of Hydrogen Chloride With Ft Ir
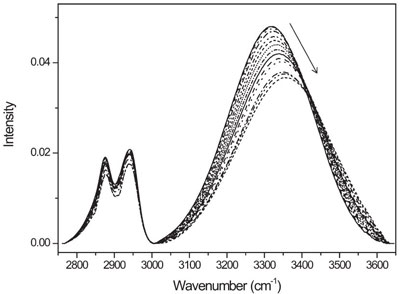
Positional Fluctuation Of Ir Absorption Peaks Frequency Shift Of A Single Band Or Relative Intensity Changes Of Overlapped Bands American Laboratory

Guide To Ft Ir Spectroscopy Bruker
